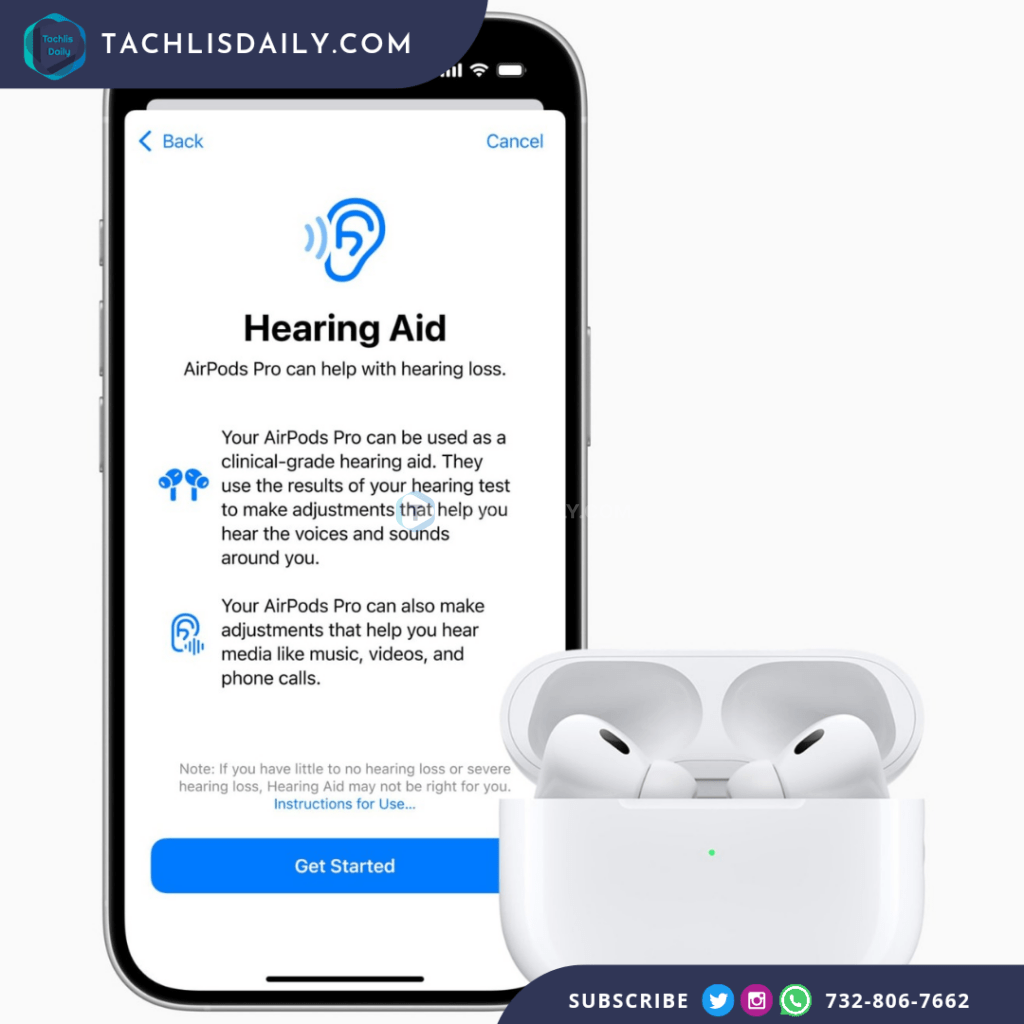
Apple’s second-generation AirPods Pro have officially received authorization from the U.S. Food and Drug Administration (FDA) to function as over-the-counter clinical-grade hearing aids. This groundbreaking feature, which will be available later this fall, has been described by the FDA as “the first over-the-counter hearing aid software device.” Apple’s latest innovation aims to make hearing aid technology more accessible, reducing both the stigma and the cost associated with traditional hearing aids.
The FDA’s approval came after a clinical study was conducted involving 118 participants who reported mild to moderate hearing loss. In the study, Apple’s self-fitting hearing aid feature demonstrated results comparable to those of professionally fitted devices. This new functionality will enable users to access hearing assistance without needing a professional fitting, offering a more user-friendly, tech-driven solution.
Apple has designed the AirPods Pro 2 not only to improve accessibility for individuals with hearing impairments but also to promote overall hearing health. Among the new features included is a Hearing Protection mode, which safeguards the user’s ears in noisy environments, such as concerts or other loud settings, by maintaining natural sound quality while protecting the listener from potential hearing damage. This feature will be activated by default when the device detects loud environments.
Additionally, the AirPods Pro 2 will introduce a personalized Hearing Test feature, allowing users to fine-tune their earbuds to match their hearing profile. The test involves listening to a variety of tones played through the earbuds, and users can interact with the test by tapping the screen when they hear a sound. The results will be recorded in Apple’s Health app, providing users with an individualized hearing profile that will optimize their listening experience through the hearing aid feature.
The ability to transform the AirPods Pro into clinical-grade hearing aids represents a major step forward for accessibility in consumer technology. According to the World Health Organization, approximately 1.5 billion people globally live with some degree of hearing loss, and Apple’s solution offers a more discreet and cost-effective alternative to traditional hearing aids. The AirPods Pro 2 will provide hearing support without the need for specialized devices, allowing users to maintain their regular earbuds while benefiting from advanced hearing technology.
This isn’t Apple’s first venture into hearing health. The company has previously integrated noise level monitoring and warnings into its products, alerting users when they are exposed to potentially damaging sound levels. With the AirPods Pro 2, Apple continues to innovate in this space, introducing features aimed at preventing hearing damage and promoting better hearing health.
The FDA’s approval of Apple’s hearing aid software falls under the De Novo classification, which is used for low- to moderate-risk devices that have no prior equivalent on the market. This classification allows for the regulation of innovative devices that introduce new technological approaches, such as Apple’s hearing aid software.
In addition to serving as hearing aids, the AirPods Pro 2 will continue to offer all the premium features users expect, including active noise cancellation, Transparency mode, and high-quality audio. By combining these advanced audio features with accessible hearing aid functionality, Apple is positioning the AirPods Pro 2 as a versatile and essential tool for millions of people around the world.
As the hearing aid feature becomes available, it could change how people access hearing assistance technology, offering a more inclusive and approachable solution. With traditional hearing aids often costing thousands of dollars, the AirPods Pro 2 could offer an affordable alternative while reducing the stigma often associated with wearing hearing aids. Apple’s focus on hearing health reflects a broader trend in the tech industry to prioritize user well-being, making advanced health features available in everyday consumer devices.
The hearing aid functionality, along with other features like the Hearing Protection mode and personalized Hearing Test, will be rolled out as part of a software update later this year. Apple is continuing to expand its commitment to health technology, and the AirPods Pro 2’s new capabilities are likely to make a significant impact on how hearing health is managed in the future.